2021 Neco Chemistry Practical Answers
Neco 2021 Chemistry Practical Questions and Answers, Neco Gce 2021 Chemistry Practical Answers, Neco Chemistry Practical Answers 2021
NECO CHEMISTRY PRACTICAL EXPO IS DONE AND DUSTED FOR MY SUBSCRIBERS ✅✅💯
DM ME NOW IF YOU ARE INTERESTED IN IT
I REMAIN THE BEST ✅✅💯
Neco Chemistry Practical Specimen 2021
Neco Chemistry Practical 2022
Neco Chemistry Practical 2021 Date
Neco Chemistry Question 2021
Neco Physics Practical 2021
Neco Timetable 2021
Wednesday 14th July 2021
Paper I: Practical – Chemistry 10:00am – 12:00noon
============================
CHEMISTRY PRACTICAL
CHEMISTRY-PRACTICAL
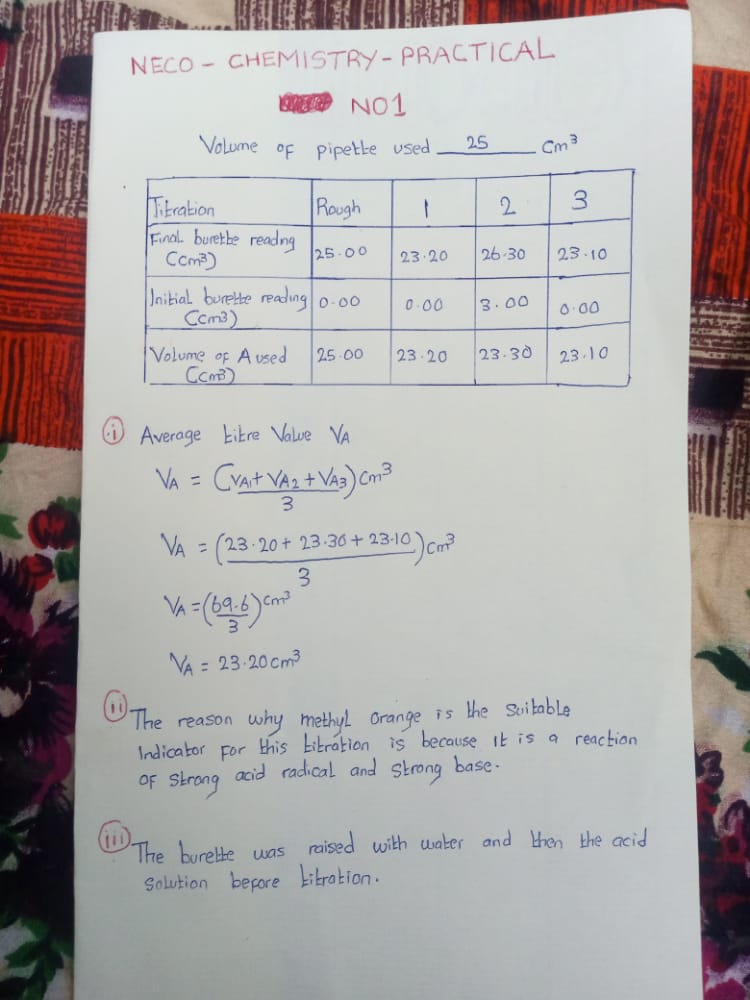 (1a)
Tabulate
Volume of pipette used=25cm3.
Indicator used= Methyl orange
Under Titration, Rough, 1st, 2nd, 3rd,
Final burette reading; |25.00| |23.40| |23.50| 23.60|
Initial burette reading; |0.00| |0.00| |0.00| |0.00|
Volume of A used; |25.00| |23.40| |23.50| |23.60|
(1ai)
Average volume of Acid used=23.40+23.50+23.60/3 =70.5/3=23.50cm3
(1aii)
This is because it is a reaction between a weak acid and strong base
(1aiii)
A funnel should be used while transferring the acid into the burette.
(1bi)
Conc of A in mol|dm³ =?
Conc of A in g|dm³ = 12.75g|dm³
Molar mass of A, NaHSO⁴ = 23+1+32+64
=120g|dm³
From.
Conc(mol/dm³) = conc(g/dm³)/molar mass
Conc =12.75/120
Conc =0.106mol/dm³
(1bii)
Con of B in mol/dm³ =?
Ca =0.106, Cb =? Va =23.50cm³, Vb =25.00cm³
na=1 , nb=1
From; CaVa/CbVb=na/nb
Cb=CaVanb/Vbna
Cb=0.106*23*50*1/25.00*1
Cb=2.5/25
Cb=0.1mol/dm³
(1biii)
Conc of B in g/dm³=?
Molar mass of B , NaOH=23+16+1=40g/mol
From; conc(g/dm³)=conc(mol/dm³)*molar mass
=0.1*40
=4.0g/dm³
(1biv)
Mass of salt formed =?
Recall mole of NaHSO⁴=?
From; mole =con(mol/dm³)*vol(cm³)/1000
Mole=0.106*23.50/1000
Mole=0.0025mol of NaHSO⁴
By proportion 1mol of NaHSO⁴ produce 1mol of Na²SO⁴ 0.0025mol of NaHSO⁴ will produce X mol of Na²SO⁴ X=0.0025*1=0.0025mol of NaSO⁴
Molar mass of Na²SO⁴=(23*2) + 32 + (16*4)
=46+32+64
=142g/mol
Recall; mole =mole/molar mass
Mass =mole * molar mass
Mass =0.0025*142
Mass =0.36g of Na²SO⁴
====================================
(2a)
TEST
C + 5cm³ of distilled water
OBSERVATION
It dissolve completely
INTERFERENCE
C is a soluble salt
(2bi)
TEST
Solution + NaOH + Heat
OBSERVATION
Effervescence occurs in which a colourless gas with pungent smell and turns red litmus paper to blue is given off.
INFERENCES
NH³ gas from NH⁴+ is present
(2bii)
TEST
Stirring rod of HCL + gas given off
OBSERVATION
a gas which gives a pop sound is given off
INFERENCES
H² is present
(2ci)
TEST
Solution C + drops of BaCL²
OBSERVATION
a white precipitate is formed
INFERENCES
CO²^-3, SO²^-4, SO²^-3, May be present.
(2cii)
TEST
Solution in C(i) + dil HCL
OBSERVATION
the white precipitate dissolve is dil HCL and effervescence occurs in which a colourless and odourless gas which turns blue litmus red and lime water milky is given off
INTERFERENCE
CO² gas from CO²^3- confirmed
====================================
(3a)
(i)Ca²+
(ii)Pb²+
(3bi)
concentrated acid can be defined as an acid formed when a large quantity of an acid dissolve in a small or little volume of water.
(3bii)
A strong acid is defined as a type of acid that ionize completely in a solution
(3c)
Activated charcoal is used as an adsorbent material
(3d)
(i)Reddish – Brown
(ii) I – red, II – brown.
====================================
Completed!. Promise Fulfilled.
(1a)
Tabulate
Volume of pipette used=25cm3.
Indicator used= Methyl orange
Under Titration, Rough, 1st, 2nd, 3rd,
Final burette reading; |25.00| |23.40| |23.50| 23.60|
Initial burette reading; |0.00| |0.00| |0.00| |0.00|
Volume of A used; |25.00| |23.40| |23.50| |23.60|
(1ai)
Average volume of Acid used=23.40+23.50+23.60/3 =70.5/3=23.50cm3
(1aii)
This is because it is a reaction between a weak acid and strong base
(1aiii)
A funnel should be used while transferring the acid into the burette.
(1bi)
Conc of A in mol|dm³ =?
Conc of A in g|dm³ = 12.75g|dm³
Molar mass of A, NaHSO⁴ = 23+1+32+64
=120g|dm³
From.
Conc(mol/dm³) = conc(g/dm³)/molar mass
Conc =12.75/120
Conc =0.106mol/dm³
(1bii)
Con of B in mol/dm³ =?
Ca =0.106, Cb =? Va =23.50cm³, Vb =25.00cm³
na=1 , nb=1
From; CaVa/CbVb=na/nb
Cb=CaVanb/Vbna
Cb=0.106*23*50*1/25.00*1
Cb=2.5/25
Cb=0.1mol/dm³
(1biii)
Conc of B in g/dm³=?
Molar mass of B , NaOH=23+16+1=40g/mol
From; conc(g/dm³)=conc(mol/dm³)*molar mass
=0.1*40
=4.0g/dm³
(1biv)
Mass of salt formed =?
Recall mole of NaHSO⁴=?
From; mole =con(mol/dm³)*vol(cm³)/1000
Mole=0.106*23.50/1000
Mole=0.0025mol of NaHSO⁴
By proportion 1mol of NaHSO⁴ produce 1mol of Na²SO⁴ 0.0025mol of NaHSO⁴ will produce X mol of Na²SO⁴ X=0.0025*1=0.0025mol of NaSO⁴
Molar mass of Na²SO⁴=(23*2) + 32 + (16*4)
=46+32+64
=142g/mol
Recall; mole =mole/molar mass
Mass =mole * molar mass
Mass =0.0025*142
Mass =0.36g of Na²SO⁴
====================================
(2a)
TEST
C + 5cm³ of distilled water
OBSERVATION
It dissolve completely
INTERFERENCE
C is a soluble salt
(2bi)
TEST
Solution + NaOH + Heat
OBSERVATION
Effervescence occurs in which a colourless gas with pungent smell and turns red litmus paper to blue is given off.
INFERENCES
NH³ gas from NH⁴+ is present
(2bii)
TEST
Stirring rod of HCL + gas given off
OBSERVATION
a gas which gives a pop sound is given off
INFERENCES
H² is present
(2ci)
TEST
Solution C + drops of BaCL²
OBSERVATION
a white precipitate is formed
INFERENCES
CO²^-3, SO²^-4, SO²^-3, May be present.
(2cii)
TEST
Solution in C(i) + dil HCL
OBSERVATION
the white precipitate dissolve is dil HCL and effervescence occurs in which a colourless and odourless gas which turns blue litmus red and lime water milky is given off
INTERFERENCE
CO² gas from CO²^3- confirmed
====================================
(3a)
(i)Ca²+
(ii)Pb²+
(3bi)
concentrated acid can be defined as an acid formed when a large quantity of an acid dissolve in a small or little volume of water.
(3bii)
A strong acid is defined as a type of acid that ionize completely in a solution
(3c)
Activated charcoal is used as an adsorbent material
(3d)
(i)Reddish – Brown
(ii) I – red, II – brown.
====================================
Completed!. Promise Fulfilled.
 (1a)
Tabulate
Volume of pipette used=25cm3.
Indicator used= Methyl orange
Under Titration, Rough, 1st, 2nd, 3rd,
Final burette reading; |25.00| |23.40| |23.50| 23.60|
Initial burette reading; |0.00| |0.00| |0.00| |0.00|
Volume of A used; |25.00| |23.40| |23.50| |23.60|
(1ai)
Average volume of Acid used=23.40+23.50+23.60/3 =70.5/3=23.50cm3
(1aii)
This is because it is a reaction between a weak acid and strong base
(1aiii)
A funnel should be used while transferring the acid into the burette.
(1bi)
Conc of A in mol|dm³ =?
Conc of A in g|dm³ = 12.75g|dm³
Molar mass of A, NaHSO⁴ = 23+1+32+64
=120g|dm³
From.
Conc(mol/dm³) = conc(g/dm³)/molar mass
Conc =12.75/120
Conc =0.106mol/dm³
(1bii)
Con of B in mol/dm³ =?
Ca =0.106, Cb =? Va =23.50cm³, Vb =25.00cm³
na=1 , nb=1
From; CaVa/CbVb=na/nb
Cb=CaVanb/Vbna
Cb=0.106*23*50*1/25.00*1
Cb=2.5/25
Cb=0.1mol/dm³
(1biii)
Conc of B in g/dm³=?
Molar mass of B , NaOH=23+16+1=40g/mol
From; conc(g/dm³)=conc(mol/dm³)*molar mass
=0.1*40
=4.0g/dm³
(1biv)
Mass of salt formed =?
Recall mole of NaHSO⁴=?
From; mole =con(mol/dm³)*vol(cm³)/1000
Mole=0.106*23.50/1000
Mole=0.0025mol of NaHSO⁴
By proportion 1mol of NaHSO⁴ produce 1mol of Na²SO⁴ 0.0025mol of NaHSO⁴ will produce X mol of Na²SO⁴ X=0.0025*1=0.0025mol of NaSO⁴
Molar mass of Na²SO⁴=(23*2) + 32 + (16*4)
=46+32+64
=142g/mol
Recall; mole =mole/molar mass
Mass =mole * molar mass
Mass =0.0025*142
Mass =0.36g of Na²SO⁴
====================================
(2a)
TEST
C + 5cm³ of distilled water
OBSERVATION
It dissolve completely
INTERFERENCE
C is a soluble salt
(2bi)
TEST
Solution + NaOH + Heat
OBSERVATION
Effervescence occurs in which a colourless gas with pungent smell and turns red litmus paper to blue is given off.
INFERENCES
NH³ gas from NH⁴+ is present
(2bii)
TEST
Stirring rod of HCL + gas given off
OBSERVATION
a gas which gives a pop sound is given off
INFERENCES
H² is present
(2ci)
TEST
Solution C + drops of BaCL²
OBSERVATION
a white precipitate is formed
INFERENCES
CO²^-3, SO²^-4, SO²^-3, May be present.
(2cii)
TEST
Solution in C(i) + dil HCL
OBSERVATION
the white precipitate dissolve is dil HCL and effervescence occurs in which a colourless and odourless gas which turns blue litmus red and lime water milky is given off
INTERFERENCE
CO² gas from CO²^3- confirmed
====================================
(3a)
(i)Ca²+
(ii)Pb²+
(3bi)
concentrated acid can be defined as an acid formed when a large quantity of an acid dissolve in a small or little volume of water.
(3bii)
A strong acid is defined as a type of acid that ionize completely in a solution
(3c)
Activated charcoal is used as an adsorbent material
(3d)
(i)Reddish – Brown
(ii) I – red, II – brown.
====================================
Completed!. Promise Fulfilled.
(1a)
Tabulate
Volume of pipette used=25cm3.
Indicator used= Methyl orange
Under Titration, Rough, 1st, 2nd, 3rd,
Final burette reading; |25.00| |23.40| |23.50| 23.60|
Initial burette reading; |0.00| |0.00| |0.00| |0.00|
Volume of A used; |25.00| |23.40| |23.50| |23.60|
(1ai)
Average volume of Acid used=23.40+23.50+23.60/3 =70.5/3=23.50cm3
(1aii)
This is because it is a reaction between a weak acid and strong base
(1aiii)
A funnel should be used while transferring the acid into the burette.
(1bi)
Conc of A in mol|dm³ =?
Conc of A in g|dm³ = 12.75g|dm³
Molar mass of A, NaHSO⁴ = 23+1+32+64
=120g|dm³
From.
Conc(mol/dm³) = conc(g/dm³)/molar mass
Conc =12.75/120
Conc =0.106mol/dm³
(1bii)
Con of B in mol/dm³ =?
Ca =0.106, Cb =? Va =23.50cm³, Vb =25.00cm³
na=1 , nb=1
From; CaVa/CbVb=na/nb
Cb=CaVanb/Vbna
Cb=0.106*23*50*1/25.00*1
Cb=2.5/25
Cb=0.1mol/dm³
(1biii)
Conc of B in g/dm³=?
Molar mass of B , NaOH=23+16+1=40g/mol
From; conc(g/dm³)=conc(mol/dm³)*molar mass
=0.1*40
=4.0g/dm³
(1biv)
Mass of salt formed =?
Recall mole of NaHSO⁴=?
From; mole =con(mol/dm³)*vol(cm³)/1000
Mole=0.106*23.50/1000
Mole=0.0025mol of NaHSO⁴
By proportion 1mol of NaHSO⁴ produce 1mol of Na²SO⁴ 0.0025mol of NaHSO⁴ will produce X mol of Na²SO⁴ X=0.0025*1=0.0025mol of NaSO⁴
Molar mass of Na²SO⁴=(23*2) + 32 + (16*4)
=46+32+64
=142g/mol
Recall; mole =mole/molar mass
Mass =mole * molar mass
Mass =0.0025*142
Mass =0.36g of Na²SO⁴
====================================
(2a)
TEST
C + 5cm³ of distilled water
OBSERVATION
It dissolve completely
INTERFERENCE
C is a soluble salt
(2bi)
TEST
Solution + NaOH + Heat
OBSERVATION
Effervescence occurs in which a colourless gas with pungent smell and turns red litmus paper to blue is given off.
INFERENCES
NH³ gas from NH⁴+ is present
(2bii)
TEST
Stirring rod of HCL + gas given off
OBSERVATION
a gas which gives a pop sound is given off
INFERENCES
H² is present
(2ci)
TEST
Solution C + drops of BaCL²
OBSERVATION
a white precipitate is formed
INFERENCES
CO²^-3, SO²^-4, SO²^-3, May be present.
(2cii)
TEST
Solution in C(i) + dil HCL
OBSERVATION
the white precipitate dissolve is dil HCL and effervescence occurs in which a colourless and odourless gas which turns blue litmus red and lime water milky is given off
INTERFERENCE
CO² gas from CO²^3- confirmed
====================================
(3a)
(i)Ca²+
(ii)Pb²+
(3bi)
concentrated acid can be defined as an acid formed when a large quantity of an acid dissolve in a small or little volume of water.
(3bii)
A strong acid is defined as a type of acid that ionize completely in a solution
(3c)
Activated charcoal is used as an adsorbent material
(3d)
(i)Reddish – Brown
(ii) I – red, II – brown.
====================================
Completed!. Promise Fulfilled.

