Neco Chemistry 2022 Answers 25th July 2022
Neco Chemistry Objective & Essay Answers 2022 for 25th July 2022 with questions 100% verified official questions and answers for senior secondary schools.
++++++++++++++++++++++
EXAM: Neco
SUBJECT: Chemistry
PIN: 711
WEBSITE: expobite.net
Kindly search EXPOBITE ANSWER PAGE on google and put the above password.
++++++++++++++++++++++
CHEMISTRY OBJ
01-10: EBAAAAAACC
11-20: BCBCACADCA
21-30: AACBDDAACD
31-40: CCACCACCDE
41-50: EDCCDDBDAC
51-60: CBACCDCBCD
Completed!
=================================
CHEMISTRY ESSAY
(1ai)
(i) KAl(SO₄)₂. 12H₂O
(ii) Na₂SO₄
(1aii)
Number of moles of sodium reascted = reacting mass/molar mass
= 3.6/23
= 0.1565moles
Number of moles of oxygen reacted = (1/4) x Number of sodium
= 1/4 x (3.6/23)
= 0.03913
Reacting mass of oxygen = Number of moles x molar mass
= 0.03913 x (16×2)
= 1.252grams
(1bi)
Air in gaseous form is first passed through caustic soda to remove CO₂. It is compressed and cooled until ut becomes a liquid at -200°C. It is then led to a fractionating column. On distillation, Nitrogen which has a lowet boiling point of -196°C is evolced first leaving behind Oxygen in liquid form. Further heating converts the liquid to a gas at -183°C.
(1bii)
(i) It is used for combustion
(ii) It is used for artificial respiration
(1biii)
This is because room temperature is warmer
(1biv)
(i) Methyl orange
(ii) Phenophthalein
(1ci)
An ion is an atom or molecule which has a net electric charge due to the loss or gain of one or more electrons.
(1cii)
HCl gas doesn’t contain ions
(1cii)
(i) Noble gases: Neon, Helium
(ii) Halogens: Iodine, Fluorine
(iii) Alkaline earth metals: Calcium, Magnesium
(1civ)
Halogens attain stable octet configuration by accepting an electron from donors Group I elements.
(1di)
A sol is a colloid where solid particles are dispersed in liquid medium.
(1dii)
Jelly
(1diii)
(i) Acid-base titrations are used to determine the percentage purity of a substance
(ii) Acid-base titrations are important to determine the number of water molecules in a hydrate.
°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°
(2ai)
A molar solution of a compound contains one mole or the molar mass of the compound in one dm³ of the solution.
(2aii)
(i) Identification of gases
(ii) Identification of acidic and metallic radicals
(2aiii)
(i) Purification of bauxite
(ii) Electrolysis of alumina
(2bi)
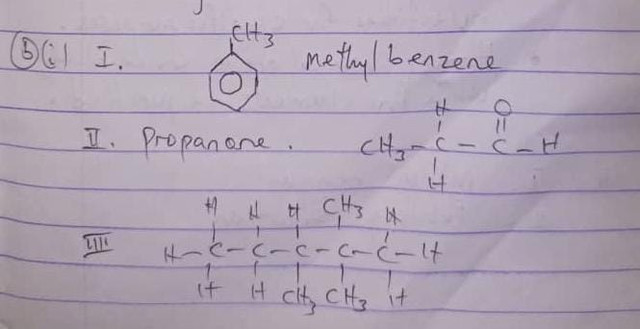
(2bii)
This is because of the presence of copper which is a transition metal
(2bii)
(i) Methanamide is a liquid while the rest amides are crystalline solids
(ii) Melting points and boiling points of aides are much higher than expected.
(2ci)
(i) Heavy chemicals are produced in very large quantities in industries While fine chemicals are produced in small quantities for specific purposes
(ii) Heavy chemicals are in a crude state While fine chemicals are purified.
(2cii)
(i) Million’s reagent
(ii) Feeling’s solution
(2ciii)
Graham’s law of diffusion states that at a constant temperature and pressure the rate of diffusion of a gas is inversely proportional to the square root of its density.
(2civ)
RO₂/RCH₄ = √(MCH₄/MO₂)
5/(200/t) = √[(12+4)/(16×2)]
5t/200 = √16/32
t/40 = 1/√2
t = 40/√2
t = (40/√2) × (√2/√2)
t = 20√2 seconds
Time taken = 28.28 seconds.


°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°
(5)

°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°°
(6a)


Neco Chemistry Answers 2022: here are the verified Neco 2022 Chemistry Objective & Essay questions and answers for SS3 students for Monday 25th July 2022. Neco 2022 Chemistry Objective & Essay Questions and Answers.
Neco Chemistry Answers 2022: EXPO: Neco Chemistry Objective & Essay Questions and Answers 2022/2023, 2022/2023 Neco EXPO ON Chemistry Objective & Essay QUESTIONS, 2022 Chemistry Objective & Essay Questions & Answers is Out.
Neco Chemistry Objective & Essay Answers 2022
Monday 25th July 2022
- Paper III & II: Objective & Essay – Chemistry – 10:00am – 1:00pm
Neco 2022 Chemistry Objective & Essay Questions and Answers
Neco Chemistry Objective & Essay Answers 2022: Welcome to the Expobite answer page where you will get verified and 100% authentic and correct Neco Chemistry Objective & Essay Answers 2022 for Monday 25th July 2022.
Examkey.net Chemistry Objective & Essay pin questions and answers for today, examkey, joberplanet, lasu-info, ceebook, noniwap, noniexpo, examspot, unn-edu, nairaland, utmeofficial, bestexamportal, loadedking.com literature Pin questions and answers for today.
Neco 2022 Chemistry Objective & Essay Questions & Answers
I know you really want to pass and get an A in your Neco 2022 Chemistry Objective & Essay examination? then follow the below to subscribe to our Neco Chemistry Objective & Essay exam runz/expo, we promise to send you both questions and answers at least 2 hours before the Neco Chemistry Objective & Essay 2022 examination.
Neco 2022 Chemistry Objective & Essay Questions and Answers Subscription Price List:
- To receive the Neco 2022 Chemistry Objective & Essay questions and answers through WhatsApp, the subscription price is ₦600 MTN airtime/recharge card.
- To have access to our Expobite Answer Page at https://expobite.net/answer-page/ where the Neco Chemistry Objective & Essay answer 2022 will be posted also using the 4 digits PIN/PASSWORD/KEY we will give you once you subscribe, the subscription price is ₦500 MTN airtime/recharge card.
- To receive the Neco Chemistry Objective & Essay 2022 questions and answers via text message/SMS, the subscription price is ₦1,000 MTN airtime/recharge card.
How to Subscribe
Kindly follow the below simple steps to subscribe to our 2022 Neco Chemistry Objective & Essay Questions and answers expo/runz for both Obj and Essay:
Choose your desired way of receiving answers
As I wrote above, we have 3 ways of sending questions and answers to our candidates, kindly choose between the 3 answer delivery methods available on our platform, ie, choose WhatsApp, PIN, or Text Message.
Transfer/Send Airtime to us.
You can either buy an MTN recharge card worth the subscription price to us or transfer it to our MTN line from your bank account through USSD or your Banking app.
Follow the below steps to successfully subscribe through the MTN recharge card:
– Buy a recharge card worth the amount of the subscription, open your WhatsApp and send the recharge card pins to us on 07062154881.
Follow the below steps to successfully subscribe through Bank airtime transfer (VTU):
– Open your bank app or USSD or go to POS and recharge our MTN line 07062154881.
We will confirm your subscription and get back to you immediately.
Neco Chemistry 2022 Answers & Questions for Practical, Neco Chemistry Objective & Essay Practical Questions and Answers 2022, 2022/2023 Neco EXPO ON Chemistry Objective & Essay QUESTIONS, Neco Chemistry Objective & Essay Questions and Answers for 2022/2023, Neco Chemistry Objective & Essay questions and answers, Neco Chemistry Objective & Essay Questions and Answers 2022/2023 Expo/Runs
I Will Send you the Correct 2022 Neco Chemistry (Objective & Essay) Questions and Answers, Neco 2022 Chemistry (Objective & Essay) Runs, Neco 2022 Chemistry (Objective & Essay) Expo, Neco 2022 Chemistry (Objective & Essay) Answers with Objective and Essay/Theory Exactly 5 hours Before the Exam. Neco 2022 Chemistry (Objective & Essay) Obj and Essay Questions & Answers
Neco 2022 Chemistry (Objective & Essay) Questions and Answers
We are pleased to inform all Neco students sitting for the 2022 Neco Chemistry (Objective & Essay) Exam that we have the complete Neco 2022 Chemistry (Objective & Essay) Expo with Questions Paper for May/June Neco Examination.
Did you know a good Neco result is your one ticket to admission to any University or Polytechnic of your choice? If you’re directed to this website pls subscribe now and smile while you write your exam.
How to Subscribe for Neco 2022 Chemistry (Objective & Essay) Answers.
Choose from the below subscription price list that best suits you, then send the exact amount as Mtn Recharge Card to us on 07062154881
TEXT MESSAGE: N1,000
WHATSAPP GROUP: N600
PASSWORD LINK: N500
See How to Make Payment For Neco 2022 Chemistry (Objective & Essay) Expo.
Send the following details:
- Your Mtn Card
- Your Subject Name
- Your Phone Number to 07062154881
Example:
8393994848484/Commerce/08123456789
Immediately we have confirmed your details, the complete 2022 Neco Chemistry (Objective & Essay) runs will be sent to you.
WHAT YOU MUST KNOW
- If you supply Neco to your students or schools and you will need Neco Chemistry (Objective & Essay) question paper and answers contact us.
- No website would post free 2022 Neco Chemistry (Objective & Essay) answers online if they got the real questions paper direct from Neco officials like us. Beware of fake websites!!
- Most of the websites posting free Neco Chemistry (Objective & Essay) expo online are posting fake answers or copied ones with little to no knowledge of how it came about.
I HOPE THE DESCRIPTION PROVIDED ABOVE IS ENOUGH TO CONVINCE YOU WE ARE 100% REAL AND LEGIT.
Neco Chemistry (Objective & Essay) Answer 2022, Neco Chemistry (Objective & Essay) 2022, Neco Timetable 2022, Neco 2022 Syllabus, Neco Time Table Download, Neco May June exam Start When, Neco May/June 2022 Registration, Is Neco Registration Still on, Neco Chemistry (Objective & Essay) Nov Dec 2022.
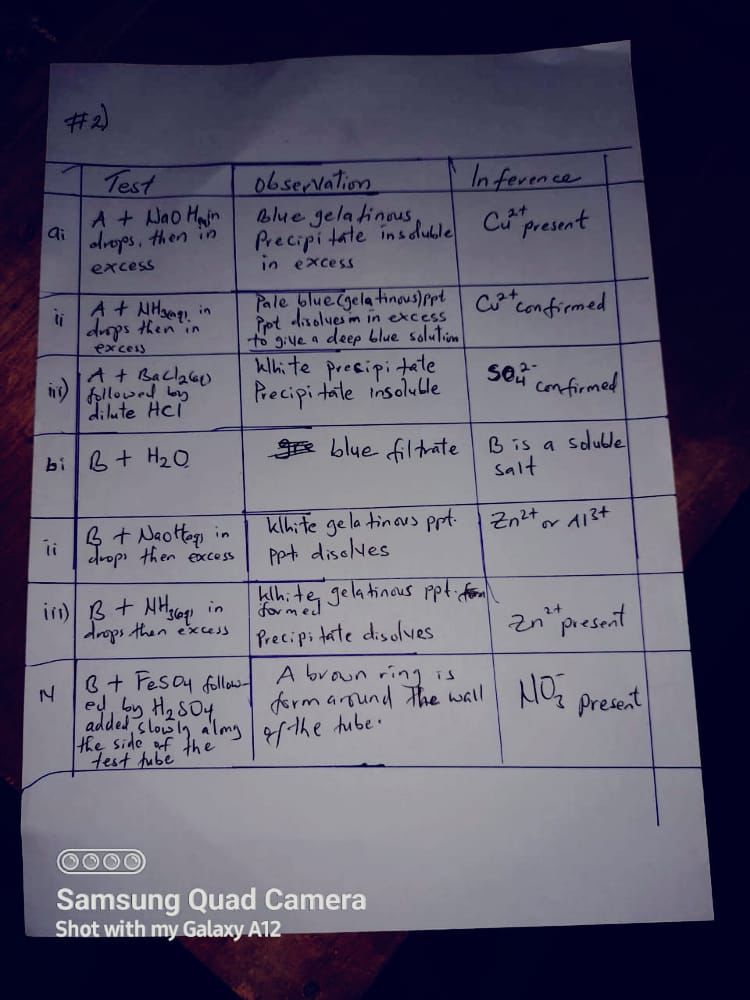
(2a)
[TABULATE]
=TEST=
C + Distilled water
=OBSERVATION=
It dissolves completely to give a light green solution.
NECO 2022 Chemistry (Essay & OBJ) Questions & Answers
NECO Chemistry Questions and Answers 2022 (100% Sure
=INFERENCE=
Soluble salt.
(2ai)
=TEST=
Solution C + NaOH in drops and in excess + Heat gently
=OBSERVATION=
A dirty green precipitate is formed which remains insoluble in excess.
Effervescence occurs in which a colourless gas with a pungent smell which turns red litmus blue is given off.
=INFERENCE=
Fe²⁺ is present.
NH₃ gas form.
NH₄⁺ is present.
(2aii)
=TEST=
Solution + BaCl₂ + dilute HCL in excess
=OBSERVATION=
A white precipitate is formed.
The white precipitate remains insoluble and gives a white dense.
=INFERENCE=
SO₄²⁻, CO₃²⁻, SO₃²⁻, is present.
SO₄²⁻ confirmed.
(2b)
Cations → Fe²⁺ and NH₄⁺
Anions → SO₄²⁻
(3a)
Sulphur Dioxide Solution Turns Acidified Potassium Chromate Solution green Where As carbon dioxide Shows no Change
(3bi)
(I) H2 ; downward displacement of water
(II) NH3; downward displacement of air
(III) HCL; upward displacement of air
(3bii)
(I) Hydrogen gas(H2) is collected by the downward displacement of water because It is insoluble in water and It form an explosive mixture with air.
(II) Ammonia gas(NH3) is collected by downward displacement of air because it is lighter than air
(III) HCl gas is collected by upward displacement of air because it is 1.28 times heavier than air.
(3c)
(i) Distillation
(ii) Filtration followed by evaporation to dryness
(3d) This is because KCl react with NaHCO₃ to form two salts






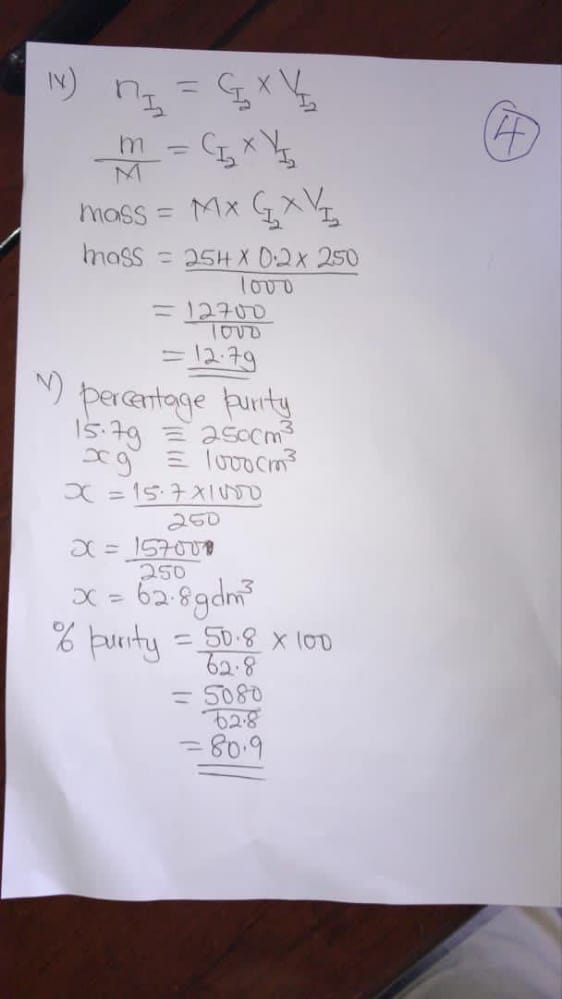
(3bi)
(I) H2 ; downward displacement of water
(II) NH3; downward displacement of air
(III) HCL; upward displacement of air
(3bii)
(I) Hydrogen gas(H2) is collected by the downward displacement of water because It is insoluble in water and It form an explosive mixture with air.
(II) Ammonia gas(NH3) is collected by downward displacement of air because it is lighter than air
(III) HCl gas is collected by upward displacement of air because it is 1.28 times heavier than air.
(3c)
(i) Distillation
(ii) Filtration followed by evaporation to dryness
1. The reaction of carbide with water gives
A. Ethyne✅✅
B. Ethene
C. Ethane
D. Ethanal
equation to reaction
C2+H2O=C2H2+02
and also the general formula for alkyne is CnH2n-2
2. The general formula of alkanones is
A. RCHO
B. RCOR’✅
C. RCOOH
D. RCOOR
Alkanones also known as ketones have the general formula RCOR’
3. A second alkanol can be oxidized to give an
A. Alkanone✅✅
B. Alkyne
C. Alkanoic acid
D. Alkanal
When an secondary alcohol is treated with acidified KMnO4, or K2Cr2O7, they yield an alkanone
4. Which of the following substances are all made by the process of polymerization?
A. Nylon and soap
B. Ethanoic acid margarine and ethanol
C. Nylon and artificial rubber✅✅
D. Soap and butane
E. Margarine and nylon
5. If an organic compound decolorizes bromine water then the compound is?
A. Saturated
B. Supersaturated
C. Unsaturated✅✅
D. A solid
E. Protonated
NECO Chemistry Practical Questions and Answers 2022 (100
NECO Chemistry Questions and Answers 2022 (100%)
6. Which of the following types of reactions takes place between C2H4 and the halogens?
A. Substitution
B. Polymerization
C. Addition✅✅
D. Saponification
E. Oxidation
7. The alkanol obtained from the production of soap is
A. Ethanol
B. Glycerol✅✅
C. Methanol
D. Propanol
E. Glycol
The alcohol we use to make fats is ‘glycerol’ When fats or oils are hydrolysed using alkaline hydrolysis, a soap and glycerol are formed:
fats (or oil) + NaOH => soap + glycerol
8. The three-dimensional shape of methane is
A. Hexagonal
B. Trigonal
C. Linear
D. Tetrahedral✅✅
E. Cubical
9. An element that can exist in two or more different structural forms which possess the same chemical properties is said to exhibit.
A. Polymerism
B. Isotopy
C. Isomorphism
D. Isomerism
E. Allotrophy✅✅
Allotropy is the existence of two or more atoms of an element having the same physical states but different physical or structural forms.
10. The hybridization of the carbon atom in ethyne is
A. Sp⁴
B. Sp³
C. Sp²
D. Sp✅✅
E. S
Ethane sp³ hybridization
Ethene sp² hybridization
Ethyne sp hybridization
Theory-Answers
(1a)
A molecular formula consists of the chemical symbols for the constituent followed by numeric subscripts showing the number of atoms of each element present while structural formula consists of symbols for the atoms connected by short lines that represent chemical bonds.
(1b)
(i) Screening effect
(ii) Size of atom
(iii) Nuclear change
(1c)
(i) The reaction must proceed both forward and backward i.e reversible
(ii) DG = O
(1d)
(i) B
(ii) It has the least ionization energy among the three
(1e)
Graham’s law of diffusion states that the rate of diffusion of a gas is inversely proportional to the square root of their densities provided other conditions remained constant
(1f)
(i) Na2SO4. Mg(NO3)2
(ii) CaCO3
(1g)
(i) Protein – Addition polymer
(ii) Perspex – Condensation polymer
(iii) Nylon – Condensation polymer
(1h)
Atomic radius can be defined as the half distance between the nucleus of two covalently bonded atoms.
(1i)
Ethanol has a higher boiling point than propane because it has stronger force of attraction than propane which has a weak van der waal’s force.
Neco Chemistry Answers 2022 Monday 25th July 2022
NECO Chemistry Questions and Answers 2022 (Theory & Obj
(1j)
(i) It aids the digestion of food
(ii) It aids the action of enzymes
(iii) It controls the availablity of nutrients
==============================
(2ai)
(i) They have the same method of preparation
(ii) They have the same chemical properties
(2aii)
(i) Ethane has a single bond while Ethene has a double bond
(ii) They belong to different homogeneous series
(iii) They have different molecular formula and structural formula
(2bi)
(i)HCl
(ii) – It is colourless
– It has an irritating odour
(iii) 2Nacl(aq) + H2SO4(aq) —> Na2SO4(aq) + 2Hcl(g)
(2ci)
Hydrocarbons are compounds having hydrogen and carbon atoms only. Examples are alkanes, alkenes, alkynes etc
(2cii)
(i) Coal
(ii) Crude oil
(2ciii)
C H
83% 100.83
83/12 17/1
6.92/6.92 17/6.92
1 2
Empirical formula = CH2
(2d)
==============================
(3ai)
H2SO4 (aq) + 2KOH(aq) —> K2SO4(aq) + 2H2O(l)
(3aii)
Given, Va = 25.0cm³, Vb = 24.0cm³, Cb = 0.15mol/dm³, Ca =?, Na = 1 , Nb = 2
Using,
CaVa/CbVb = Na/Nb
Ca = CbVbNa/VaNb
Ca = 0.15 × 24.0 × 1/25.0×2
Ca = 3.6/50 = 0.072mol/dm³
(3bi)
(i) 2Mg(s) + CO2(g) —> 2MgO(aq) + C(g)
(ii) This is because magnesium can reduce carbon (iv) oxide to black carbon and producing magnesium oxide
(3bii)
Mg(NO3)2
M of Mg(NO3)2 = 24+(2×14) + (6×16)
= 24 + 28 + 96
= 148g/mol
% of N = 28/148 × 100/1
N = 18.9%
(3ci)
(i) Reaction with KMnO4
(ii) Reaction with bromine water
(3cii)
It turns them colourless
(3d)
Boil the vegetable oil in sodium hydroxide solution, it breaks down releasing the organic acid and the alkanol. The process is known as saponification. The organic ester is immediately neutralized by the sodium hydroxide solution to form the sodium salt of the organic acid which is soap
=================================
(4ai)
(i) Graphite – Hexagonal
(ii) Diamond – Octahedral
(4aii)
Diamond us hard because of the strong force of attraction that hold them together and it can not conduct electricity because all the available electrons are used for bonding while Graphite is soft because it’s particles are in layers and can conduct electricity because not all the electrons are used for bonding
NECO Chemistry Questions & Answers 2022 (OBJ-Essay
NECO 2022 Chemistry Questions and Answers (Essay and
(4bi)
(i) It helps to remove dissolved gases and oxidizes dissolved metals
(ii) It helps to remove objects such as rags, paper, plastics, and clogging of downstream equipment
(iii) It reduces particle concentration in water and minimizes the need for coagulation and flocculation
(4bii)
(i) Calcium tetraoxosulphate (vi)
(ii) Calcium hydrogen trioxocarbonate
(4biii)
(i) Filtration
(ii) Boiling
(4biv)
It has a sweet taste and it builds the shells of lower organisms
(4ci)
The ore (SnO2) is wasted out of the ground with water, powdered, washed and roasted to drive off oxides sulphur, arseric and antimony. The roasted one is now reduced by heating with coke in a reverberatory furnace and molten tin is tapped off from the bottom of the furnace. The tin obtained is impure and it is remelted on a slopping furnace in which the tin melts and run its moulds. The tin obtained is 99.9% pure
Neco Chemistry (Objective & Essay) Answer 2022, Neco Chemistry (Objective & Essay) 2022, Neco 2022 Chemistry (Objective & Essay), Alternative to Chemistry (Objective & Essay) Past Questions Pdf.
NECO Chemistry Answers 2022 [Theory & Objectives]
2022 NECO EXAM Chemistry Questions and Answers


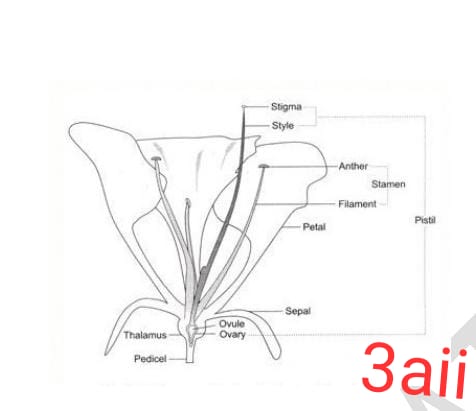

Thank you very much